Sugarcane is the principal raw material used for the manufacture of plantation white sugar. In India, sugar is refined using the the Double Sulphitation method.
This is the most widely used and cheapest process of refining sugar as compared to other available process. However the major drawback of the Sulphitation proecsss is that Sulphur enters sugar during its refining process. The end product that we consume contains sulphur dioxide approximately to the extent 20 to 70 ppm depending on the process applied as also condition of the plant and the techniques applied for the manufacture of sugar(Refined or Normal process).
As per IS1 grade of white refined sugar, the maximum permissible limit is 70 ppm whereas International standard (refined) is about 10 ppm.Normally, outside India refined sugar is produced. Perhaps this limit has been prescribed keeping in view the impact of sulphur on human health.

Originally the sulphate (SO3) content2 in sugarcane juice varies from 0.11% to 0.52%.Normallyphosphates, silica and magnesium are partially removed by clarification. However, potassium, sodium, chloride and low concentration of sulphate are not removed and these constituents tend to concentrated3 with onward processing and even pass to the bagging stage.
Entry of Sulphur Di Oxide into plantation white Sugar
In India double sulphitation process is followed for the refining of white plantation sugar as double carbonation process has since been dispensed with. Inthis process, continuous liming and sulphitation is done in a reaction tank for which continuous supply of sulphur dioxide is obtained from a continuous sulphur burner by burning sulphur in presence of dry air. The chemical reaction takes place as sulphur dioxide + oxygen producing sulphur dioxide4.
At this point besides sulphur dioxide small quantity of sulphur trioxide is also produced..During reaction atthe liquid phase in the continuous reaction tank small amount of soluble sulphate is also produced besides insoluble calcium sulphite in bulk. This calcium sulphite in later stage helps in removing various impurities including colloidal matters. After clarification of cane juice, un-sulphured syrup obtained from evaporator is again sulphited in a syrup sulphiter. Here the excess sulphur dioxide reacts with water forming sulphurous acid. Sulphur dioxide is commonly known as sulphurous acid i.e, the anhydride of the sulphurous acid5.
The presence of this sulphurous acid in sugar increases the sulphur dioxide content of sugar as it is present in sugar as anhydride of sulphurous acid. Keeping in view the health hazard, Bureau of Indian Standard: 5982-1970 has kept maximum sulphur dioxide contentlimit as 70 ppm because it is within the safe tolerance limit. For plantation white sugar limit has been fixedfor 70 ppm and for refined sugar it has been fixed as 20 ppm whereas International Standard has been fixed at 10 ppm for refined sugar.
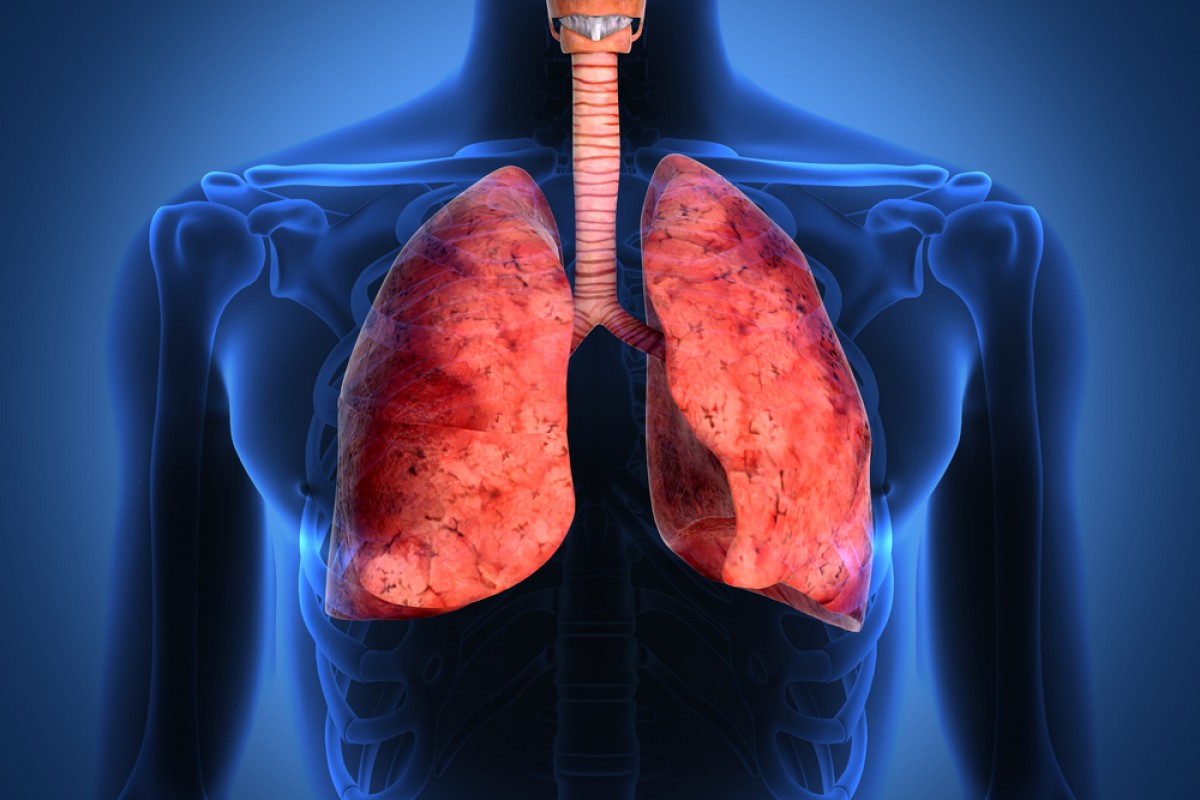
Intake of sulphur dioxide causes or may cause following health hazards6,7,8,10
(i) Intake of sulphur dioxide beyond prescribed limit causes irritation of respiratory tissues;
(ii) Sulphur dioxide causes respiratory diseases like bronchitis and asthma;
(iii) Causes breathing difficulty;
(iv) Severe airway obstruction;
(v) Causes irritation of the upper respiratory tract which sometimes may cause pneumonia9;
(vi) Causes change in breathing;
(vii) Asthmatic patients are more susceptible to sulphur dioxide consumption;
(viii) Sulphur dioxide consumption may cause allergic reaction to asthmatic patients;
(ix) Sulphur dioxide on consumption coming in contact with water forms sulphurous acid which inhibits muconcilary transport;
(x) Bisulphite ion produced when sulphur dioxide reacts with water is likely to be the main initiator of sulphur dioxide induced bronchoconstriction;
(xi) Excess intake of sulphur dioxide may cause sneezing, sore throat, wheezing, shortness of breath, chest tightness and suffocation;
Due to above problems consumers of sugar are advised to take sulphur free sugar as prevention is better than cure.
Attributed to:
Dr S C Ray, Eminent Sugar expert
References:
1. IS: 5982-1970
2. Cane Sugar Hand Book- by James C.P.Chen, Eleventh Edition
3. Cane Sugar Hand Book- by James C. P. Chen, Eleventh Edition
4. Principles of Sugar Technology- by Pieter Honig.
5. Principles of Sugar Technology- by Pieter Honig
6. Medical Management Guidelines for Sulphur Dioxide
7. Canadian Centre for Occupational Health and Safety.
8. Health Effect of Project Shad Chemical agent Sulphur Dioxide
9. Poisons, properties, chemical identification, symptoms and emergency treatment-by Vincent J. Brookes andMorris B. Jacobs.
10. .Foundation Course in Science and Technology- Indira Gandhi National Open University School of Science.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.