British-Swedish pharma giant AstraZeneca and US pharmaceutical major Novavax have told legislators that they are ready to scale up their production of Covid-19 vaccines in partnership with the Serum Institute of India (SII).
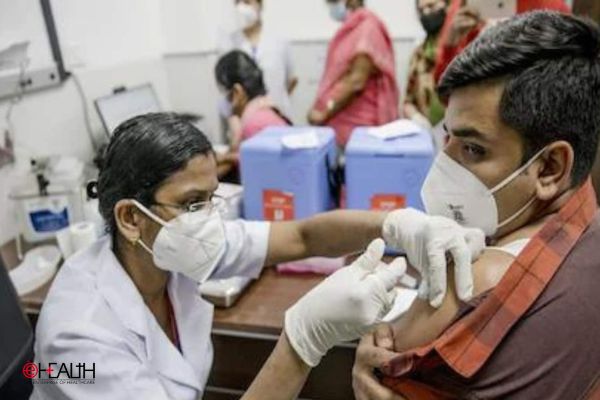
Located in Pune in Maharashtra, the SII is the world’s largest vaccine manufacturer by volume. SII is manufacturing the coronavirus vaccine developed by AstraZeneca and Oxford University, which is known locally as Covishield. Covishield was granted emergency use listing by the World Health Organization (WHO) in February, allowing it to be supplied to low and middle-income countries around the world.

“Together with our partner, the Serum Institute of India, we plan to supply over 300 million doses to 145 countries through COVAX in the first half of 2021 as part of our global and equitable access pledge,” Ruud Dobber, president of AstraZeneca’s biopharmaceuticals business said.
“The majority of this supply will go to low and middle-income countries,” Dobber told members of the US House Energy and Commerce Committee’s oversight and investigations subcommittee during a Congressional hearing on ‘Pathway to Protection: Expanding Availability of Covid-19 Vaccines’.
The purpose of the hearing was to examine manufacturers’ ongoing efforts to develop and scale-up the production of Covid-19 vaccines in the US.

Also read: India wants Serum Institute to lower price of AstraZeneca shot
The COVAX or Covid-19 Vaccine Global Access programme is run by Gavi, the Vaccine Alliance, and WHO in partnership with developed and developing country vaccine manufacturers.
Dobber said the company’s agreement with the US government covers the development and supply of 300 million doses of its vaccine, should it receive authorisation. The cost of the doses and the dose agreements will provide no profits for AstraZeneca.
Its vaccine has received conditional marketing or emergency authorisation in more than 50 countries, and recently, the WHO listed its vaccine for emergency use against Covid-19, he told the legislators.
“Studies conducted today indicated our vaccine is well tolerated and effective. And just this week, we were encouraged to see the first real-world evidence from over 5.4 million subjects in Scotland demonstrating a risk reduction of Covid-19 related hospitalisations by 94% after the first dose of our vaccine,” he asserted.
John Trizzino, executive vice president of Novavax, also gave a similar assurance to members of the Congressional Committee. “Scale-up has not begun yet in large scale but we do have a global capacity both with our existing Novavax facilities in the US, our partners in India’s Serum Institute, and we believe that we could scale up that new strain very quickly,” Trizzino said in response to a question.
Covishield is less expensive compared to some of the other vaccines being used — such as the ones from Pfizer-BioNTech and Moderna. It also does not need to be stored in ultra-low temperatures, which makes it suitable for use in many developing countries that lack the necessary storage infrastructure.
In his written testimony, Trizzino said once all of its capacity comes online globally, which it expects to happen in the mid-point of this year, Novavax will have a global capacity to produce approximately two billion doses per year, roughly 150 million doses per month.
“This includes all of our partners, including the Serum Institute of India. During the pandemic, we believe that the federal government and state and local jurisdictions are in the best position to determine how doses are distributed and allocated. We will work with government leaders to ensure that those who need a vaccine get it,” he said.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.