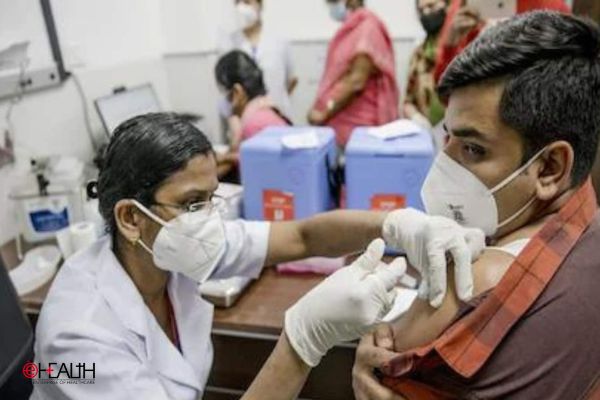
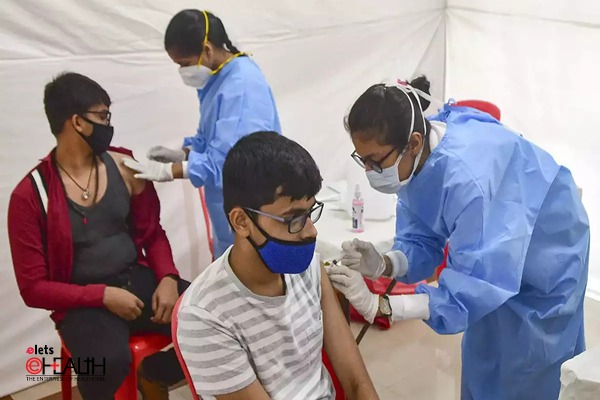
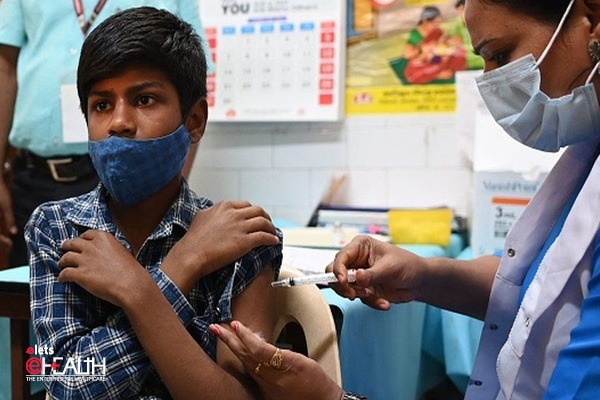
After the latest decision to approve the vaccine jointly produced by UK-Swedish biopharmaceutical company AstraZeneca and Oxford University as well as by Bharat Biotech for emergency use in India to immunise people against the COVID-19 virus; many experts’ question the foundation of these approvals and reasons why other candidates such as Sputnik V and Pfizer were neglected in the process.
According to DCGI, Oxford-AstraZeneca Covid vaccine shows an average 70% effectiveness in preventing the virus. To which Dr. Debkishore Gupta, Dr. Debkishore Gupta- Consultant Clinical Microbiologist and Head of Infection Control, CK Birla Hospitals asserts: “This is the average efficacy between the normal group which received two full doses (62%) and half-dose and full dose (90%). India approved a full-dose regimen. Based on the full dose regimen for both shots that got approved, AZ had showed 62% efficacy based on their own paper submitted to the Lancet magazine; this is much lower than any other vaccine that has been approved in the world but yet to be approved by DCGI.” Dr. Gupta goes on to add, “Trial is not yet complete in India. According to The Lancet- ‘In participants who received two standard doses, vaccine efficacy was 62.1% and in participants who received a low dose followed by a standard dose, efficacy was 90.0%. Overall vaccine efficacy across both groups was 70.4%.’ As India will possibly go by 2 full doses regimen, efficacy should have been quoted at 62.1%.

The mention of the “overall” efficacy at 70% in this case is misleading.Moreover, AstraZeneca uses one and the same component for both inoculations, the Russian vaccine uses two different ones in two separate inoculations. Using the latter approach could prove more efficient in achieving a longer-lasting immune response.” There has been ambiguity over AstraZeneca vaccine, ever since the data was published in the Lancet journal in the month of November last year proving that two full shots were 62.1% effective. AstraZeneca later announced plans to collaborate with Sputnik V whose efficacy showed to be 91.4% based on analysis of the data of the final control point of clinical trials. The partners will assess the immunogenicity and safety of the combined use of one of the components of the Sputnik V vaccine developed by the Gamaleya Center, and one of the components of the AZD 1222 vaccine, developed by AstraZeneca and the University of Oxford. Such a combination should ultimately boost the efficacy the efficacy of AstraZeneca’s vaccine to over 90%.
Also read: ‘Covid-19 vaccine will be equally effective against new corona mutant’
Meanwhile, the makers of Sputnik V took to Twitter and shared, “Indian regulator recommends for approval full-dose regimen of AstraZeneca’s vaccine with efficacy of 62.1% based on phase 3 clinical data. Sputnik V is working on clinical trials with AZ to increase the efficacy of AZ vaccine to over 90%.” Dr. Ashish Bharadwaj, Dean and Executive Director, Jindal School of Law adds: “Despite the logistical, financial and administrative requirements of this exercise and with the rampant spread of the virus, primacy must be placed on the candidate vaccine that has the higher proven efficacy and lowest probability for serious side-effects. AstraZeneca has reported in The Lancet an efficacy rate for its twin-shot vaccine that is lowest among the top contenders.” “The approval of the Bharat Biotech and AstraZeneca vaccines on the primary basis will kick start the vaccination campaign. However, in the longer run keeping in mind the demand of the largely populated country like India and India’s promises to other countries, approval of other vaccines such as Pfizer and Sputnik V which are in the queue, would also be very critical.

Other players are equally important and in order to cater to the domestic and global demand, mass vaccination is the key,” says Dr. Gajendra Singh, Public Health Expert. However, Dr Rahul Pandit, Director-Critical Care, Fortis Hospitals, Mumbai & Member of Maharashtra’s COVID19 task force, has lauded the move. He said: “We have begun the new year on an extremely positive note as we get ready for the historic COVID19 vaccine roll out. Serum Institute of India and Oxford-AstraZeneca have received the Emergency Use Authorization (EUA) for the COVID19 vaccine from the Subject Expert Committee (SEC) yesterday. Their recommendation has been sent to Drugs Controller General of India with conditions, for approval, the final nod is awaited. This is a big step for India. Vaccinating such a huge population in a diverse geography is a challenge, but we have a strong national Pediatric Immunization Program in place, its amalgamation with our robust election progress will help with the vaccine deployment. The vaccine is a vector-based vaccine which has gone through three significant trial stages with 70% efficacy rate. I would emphatically say that the vaccine has gone through rigorous trials, and followed processes mandated for vaccine-making.
While it has been rolled out within a year, there is absolutely nothing to worry about.” He has suggested that some people who are allergic and have compromised immune system need doctor’s approval first. “There are some people who have severe drug allergies (leading to anaphylaxis reaction) and those with compromised immune systems who need to first take approval from their doctors before they take the vaccine shot. With the doctor’s go-ahead, these two groups should certainly get the vaccine. As of now, no data is available for vaccination of children and pregnant patients; an advisory in the UK suggested that pregnant patients can take the vaccine only if they are at a high risk of contracting the disease, and that it should be taken as late in pregnancy as they can. Also, making the vaccine available free of cost for Indians is a huge step taken by the government. It gives us the much need boost in confidence. Healthcare leaders such as Union Minister of Health & Family Welfare Dr. Harsh Vardhan strongly advocated for the vaccine, and assured people that the vaccine has gone through rigorous efficacy checks, and is absolutely safe to take,” Pandit said.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.