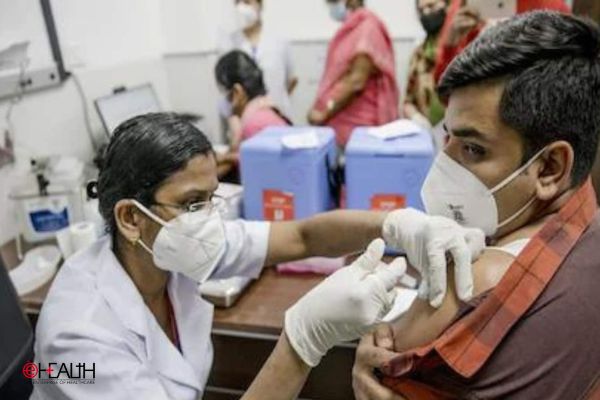
As India prepares to start vaccination drive against COVID-19 in couple of weeks, most probably in January next year, all eyes seem to be curious to know the name of the vaccine to get first approval from regulator for emergency use authorization.
Reportedly, India banks upon the UK which is likely to give its nod to the Oxford COVID-19 vaccine next week. Once the vaccine gets approval in UK, Indian regulator will decide on giving emergency use authorization to the Serum Institute that is manufacturing the shots, PTI quoting sources said.

Once the UK drug regulator gives its approval to the Oxford vaccine, the expert committee on COVID-19 at the CDSCO will hold its meeting and thoroughly review the safety and immunogenicity data from the clinical evaluations conducted abroad and in India before granting any emergency authorisation for the vaccine here, official sources was quoted saying by the report.
The process of granting emergency use approval for Bharat Biotech’s COVID-19 vaccine ‘Covaxin’ may take time as its phase 3 trials are still underway, while Pfizer is yet to make a presentation. “Going by this, Oxford vaccine ‘Covishield’ is likely to be the first to be rolled out in India,” the PTI report quoting source said.
Serum Institute of India (SII) last week also had submitted some additional data required by the Drug Controller General of India (DCGI), the sources said. Amid fears about the mutated variant of SARS-CoV-2 detected in the UK, government officials recently said that it will have no impact on the potential of emerging vaccines that are being developed in India and other countries. Bharat Biotech, Serum Institute of India (SII) and Pfizer had applied to the Drugs Controller General of India (DCGI) seeking emergency use authorisation for their COVID-19 vaccines early this month.

The subject expert committee (SEC) on COVID-19 of the Central Drugs Standard Control Organisation (CDSCO) on December 9 had sought additional safety and efficacy data for COVID-19 vaccines of SII and Bharat Biotech after deliberating upon their applications. While considering SII’s application, the SEC had recommended that the firm should submit an updated safety data of phase 2 and 3 clinical trials in the country, immunogenicity data from the clinical trial in the UK and India, along with the outcome of the assessment of the UK Medicines and Healthcare products Regulatory Agency (MHRA) for grant of EUA.
The Pune-based SII, the world’s largest vaccine manufacturer, has made a collaboration with the University of Oxford and AstraZeneca to manufacture the vaccine.
The SII has already manufactured 40 million doses of the vaccine, under the at-risk manufacturing and stockpiling licence from the DCGI, officials recently had said.
(With inputs from PTI)
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.