

Director, Health Safety and Regulation, Government of Himachal Pradesh
Mandated to ensure effective implementation and monitoring of various Acts applicable to the health sector in Himachal Pradesh like Environment (Protection) Act, the Mental Health Act, Drugs and Cosmetic Act, management of bio-medical waste, human organ transplantation, etc, the Directorate of Health Safety and Regulation, Government of Himachal Pradesh has taken various initiatives in the area of ease of doing business, says its Director Captain (Retired) Raman Kumar Sharma in an interview with Rajbala of Elets News Network (ENN).
How the Directorate of Health Safety and Regulation ensures effective implementation and monitoring of various Acts applicable to the health sector in Himachal Pradesh?

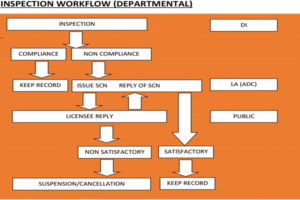
The Directorate of Health Safety and Regulations ensures effective implementation and monitoring of various Acts through its enforcement staff. As far as enforcing the Drugs and Cosmetics Act is concerned, there are drug inspectors, assistant drug controllers and the state drugs controller who effectively implement the various provisions of the Act. The Directorate also provides grants and renewal of licenses, drawing samples and inspection of sales and manufacturing units across the State.
 The Directorate of Health Safety and Regulation has structured the process of regulating various grants related to manufacturing, wholesale and retail drug licenses, pharmacy licenses, etc. The Directorate closely monitors and provides adequate arrangement for the prescribed storage of drugs in order to maintain potency during the period of shelf-life of the drugs.
The Directorate of Health Safety and Regulation has structured the process of regulating various grants related to manufacturing, wholesale and retail drug licenses, pharmacy licenses, etc. The Directorate closely monitors and provides adequate arrangement for the prescribed storage of drugs in order to maintain potency during the period of shelf-life of the drugs.
What is the procedure and timeline for providing grants and renewal of drug manufacturing in the State?

The timeline for grants and renewal of Drugs manufacturing licenses in the State is 21 days after the submission of online application on portal to the final dispense of an approval certificate. After the application for grants or renewal of licenses is received by the licensing authority, documents are scrutinised and the drug inspector is asked to conduct the physical inspection of the proposed premises. The drug inspector inspects the premises and submits a report to the licensing authority, which further grants/renews/rejects the license as per the report.
What initiatives or strategies have been adopted to promote ease of doing business in the healthcare sector?
Under the ease of doing business initiative, online receiving and disposal of applications are helping to create good business environment in the healthcare sector. Our State is under the process of accepting manufacturing applications online and their disposal will be through software only. This proposal is in the pipeline.
THE TIMELINE FOR GRANTS AND RENEWAL OF DRUGS MANUFACTURING LICENSES IN THE STATE IS 21 DAYS AFTER THE SUBMISSION OF ONLINE APPLICATION ON PORTAL TO THE FINAL DISPENSE OF AN APPROVAL CERTIFICATE.
Baddi has become a major manufacturing hub for Indian and multinational pharma companies. Do you have any other such project to promote pharma manufacturing in Himachal Pradesh?
 Baddi has become one of the major manufacturing hub for pharmaceutical companies in the State. Many R&D plants, pharmaceutical productions “ mainly formulations “ have been established there.
Baddi has become one of the major manufacturing hub for pharmaceutical companies in the State. Many R&D plants, pharmaceutical productions “ mainly formulations “ have been established there.
We are in discussions to expand pharma manufacturing industry in other parts of the State as well. Other than Baddi, there are places like Solan, Parwanoo, Tahliwal, Kala Amb, Paonta Sahib and Sansarpur Terrace where manufacturers have shown interest to set-up pharmaceutical units.
In addition to that, a world-class laboratory for testing of drugs will be established at Baddi in coming years.
Tell us about e-health initiatives and various government schemes implemented by the State.
Online, or we can say technology presence, has brought transparency in the functioning of internal system and facilitated close monitoring by the management and authorities to achieve uniformity and standardisation in the procedures and processes of manufacturing, issuing of pharma licenses, wholesale retail licenses, etc.
In terms of online initiatives, the grants or renewal of sales licenses has already been made online. Under our e-health initiative, the manufacturing licenses will be online very shortly. This facility will help companies in obtaining licenses or renewals in shortest span of time.
We look forward to implement user-friendly application modules to increase efficiency in the industry of the State in near future.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.












