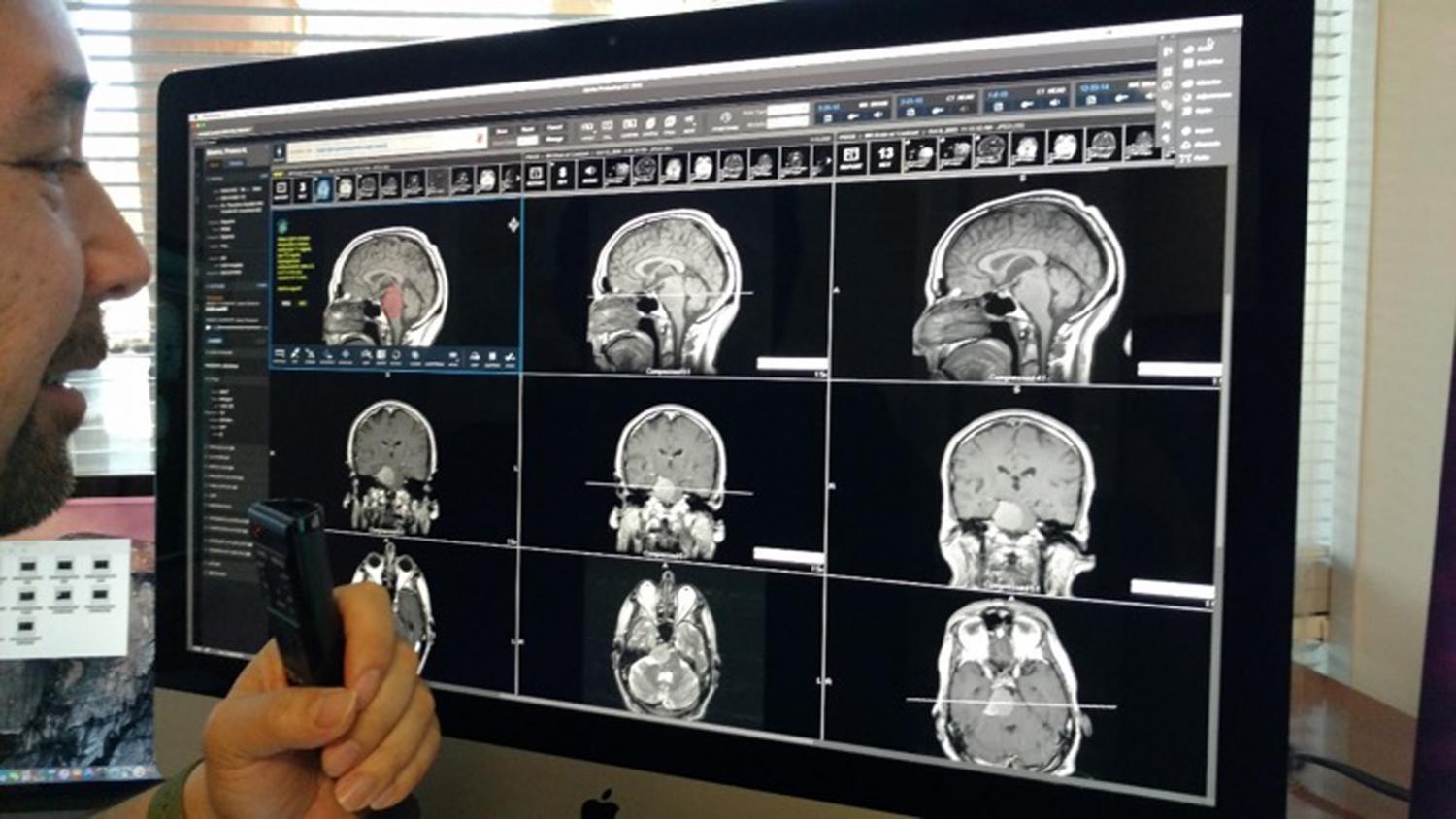
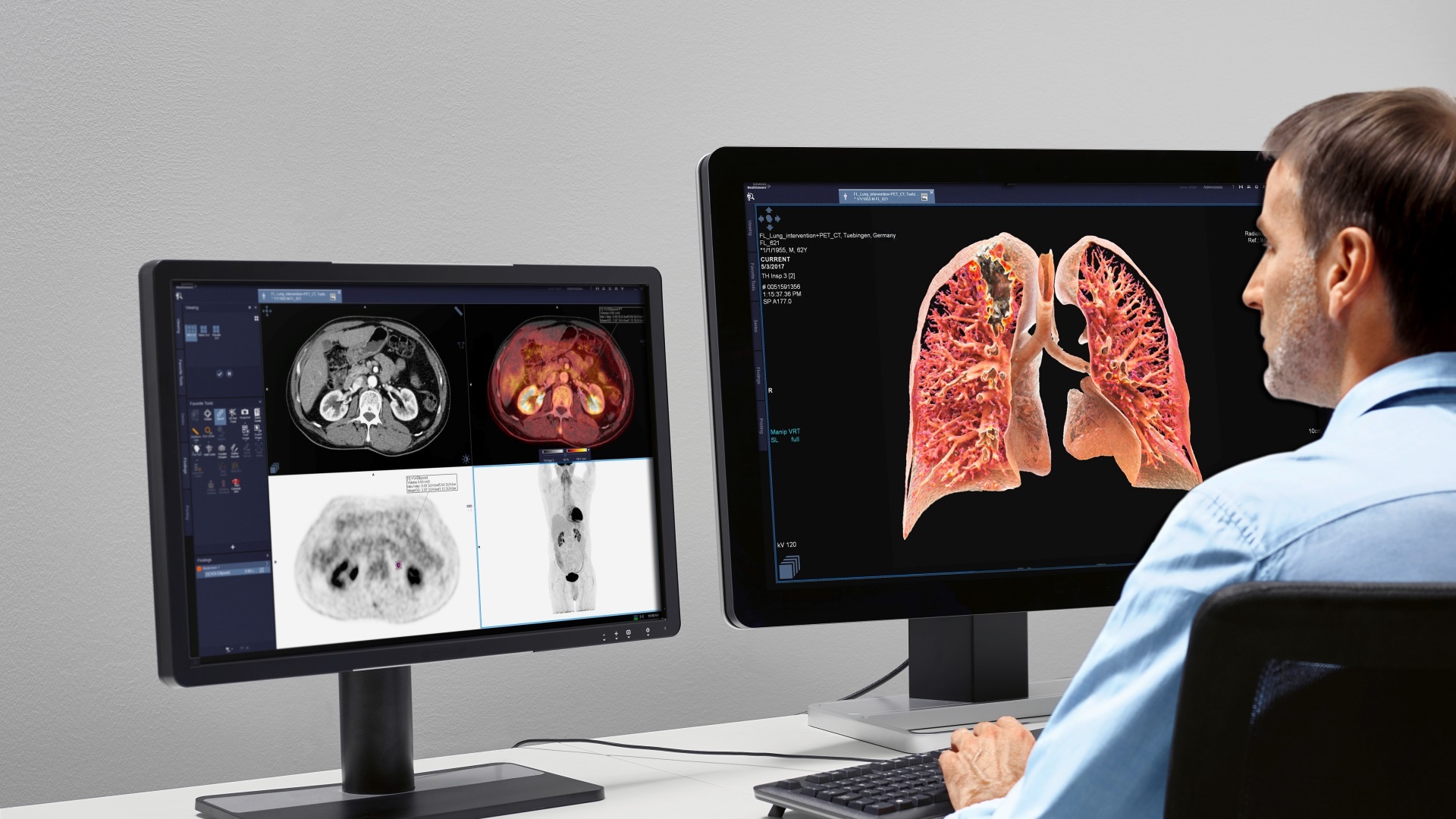
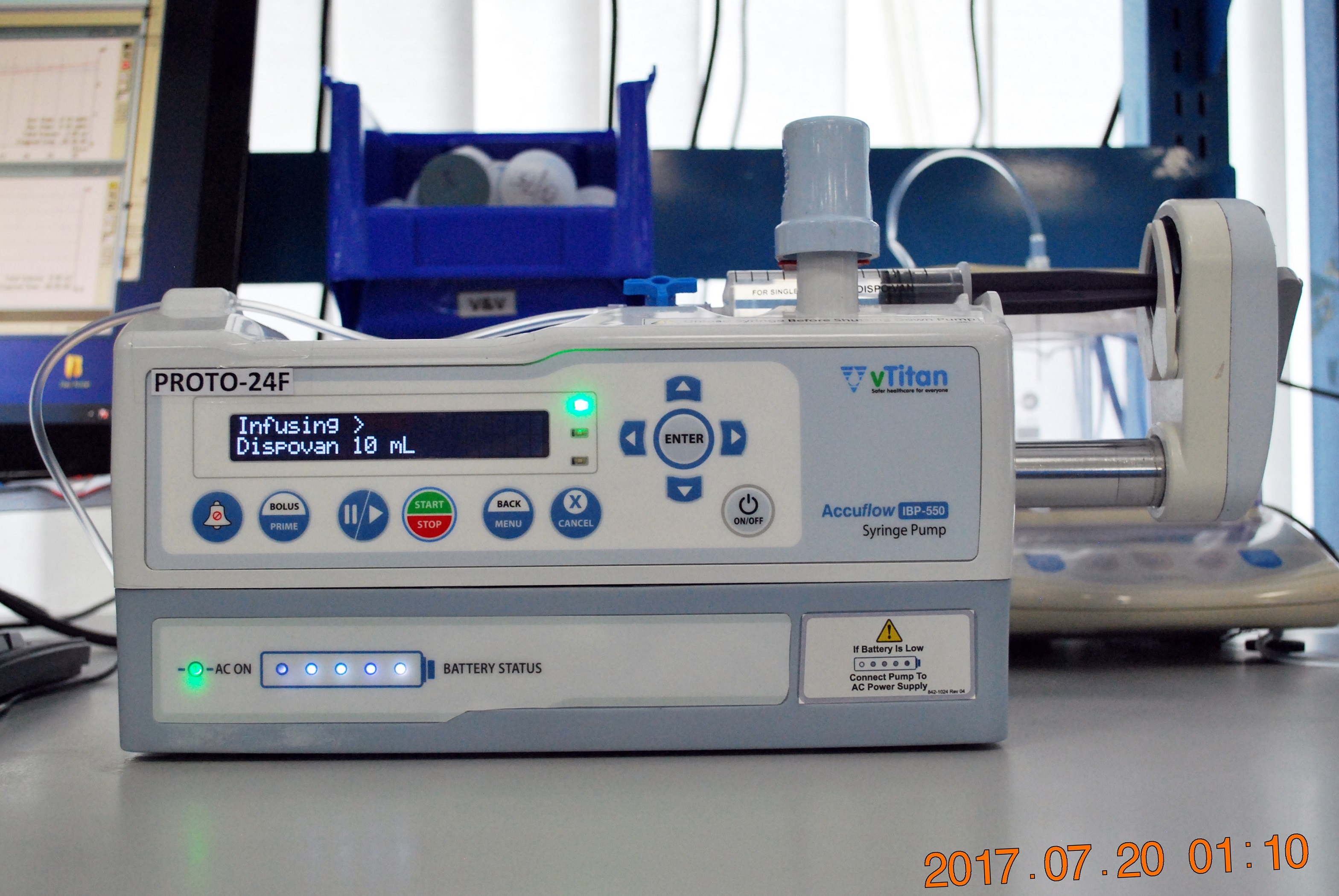
The US Food and Drug Administration (FDA) has taken steps to strengthen the approval process for radiotherapy devices following multiple errors. In a letter to the device manufacturers, the FDA identified issues from recent analysis of more than 1,000 reports of errors, which could be fixed by incorporating additional safeguards. The FDA will no longer allow new radiotherapy equipment to enter the market via streamlined approval processes that sometimes involved the use of third-party reviewers. Of the devices involved in the error reports, linear accelerators accounted for 74 percent, radiation therapy treatment planning systems accounted for 19 percent and ancillary devices, such as radiation therapy simulators, accounted for 7 percent. FDA’s Center for Devices and Radiological Health director Jeffrey Shuren, who signed the letter, said the FDA plans to hold a public workshop on radiation therapy treatment planning, linear accelerators and ancillary devices. The public workshop includes the topics related to new safeguards and other special controls to improve safety; possible changes in premarket device testing to provide appropriate assurances of safety and effectiveness, particularly for software.

Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.