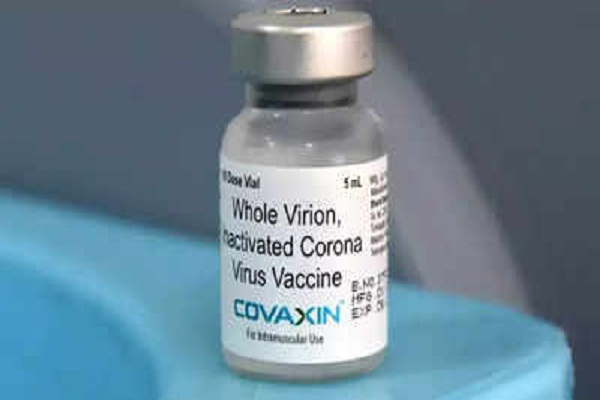
The Emergency Use Listing (EUL) of Covaxin, manufactured by Bharat Biotech, is likely to be discussed today by the Technical Advisory Group (TAG) of the World Health Organization (WHO). TAG had asked for additional clarifications from Bharat Biotech and is likely to give its final decision on EUL risk-benefit assessment for global use of covaxin.
The WHO clearance will be a significant step in the acceptance of the vaccine by foreign countries and make overseas travel easy for people who have taken this vaccine.

As shared before, data submitted by Bharat Biotech had revealed that Phase III clinical trials of Covaxin had demonstrated an efficacy rate of 77.8 per cent. It is the second-most widely used coronavirus vaccine in India after Serum Institute of India’s Covishield. The expression of interest for EUL was submitted in April by Bharat Biotech. Covid-19 vaccines manufactured by Pfizer-BioNTech, US pharma majors Johnson & Johnson, Moderna, China’s Sinopharm and Oxford-AstraZeneca have been granted emergency use listing by WHO till now.
Further, 11,903 new cases of Coronavirus (Covid-19) disease were reported in the country and 311 Covid-19-related deaths in the past 24 hours. 14,159 people recovered from the Covid-19 in the past 24 hours. Total Covid-19-related recoveries in the country have touched 3,36,97,740.

Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.












