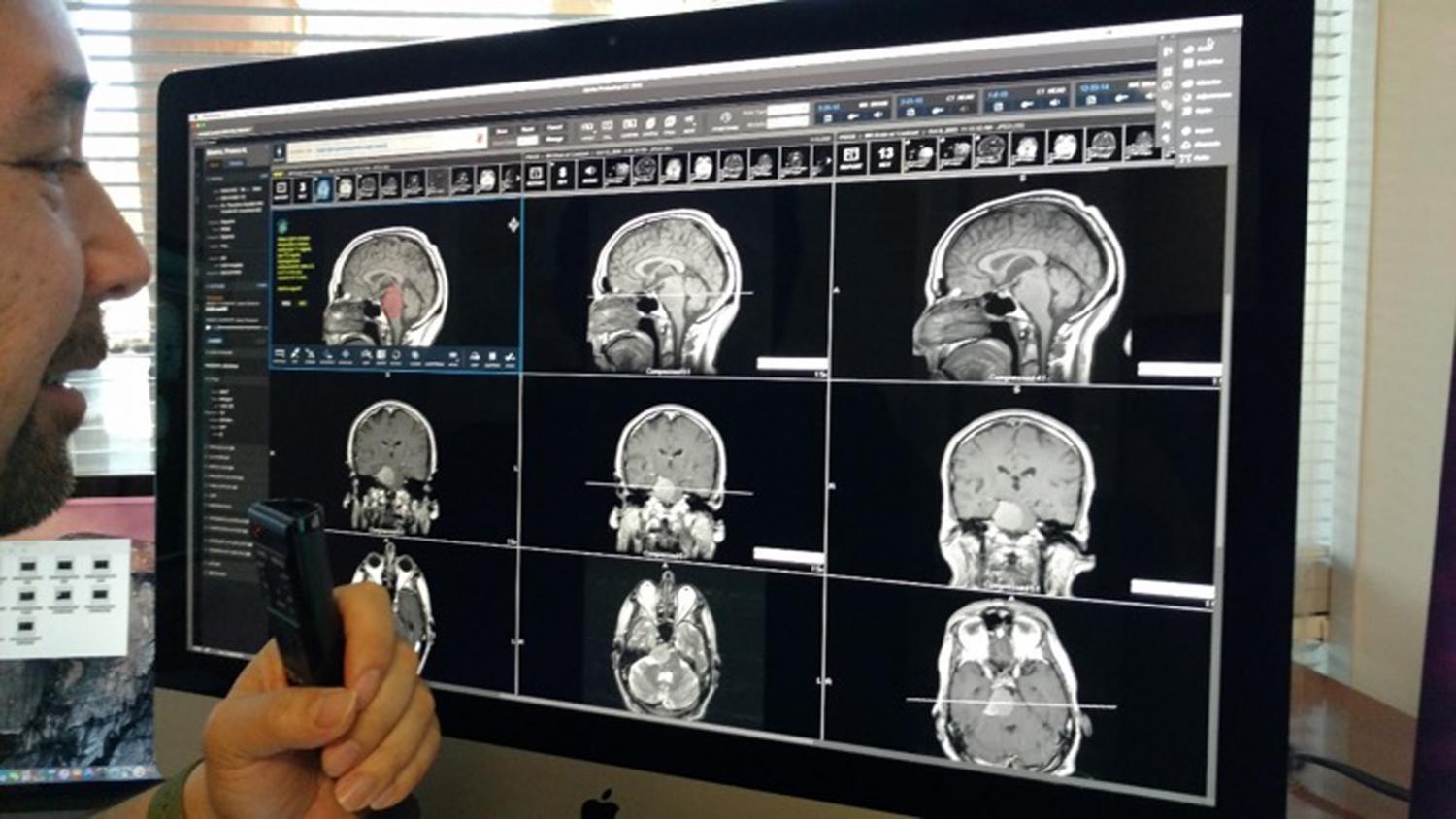
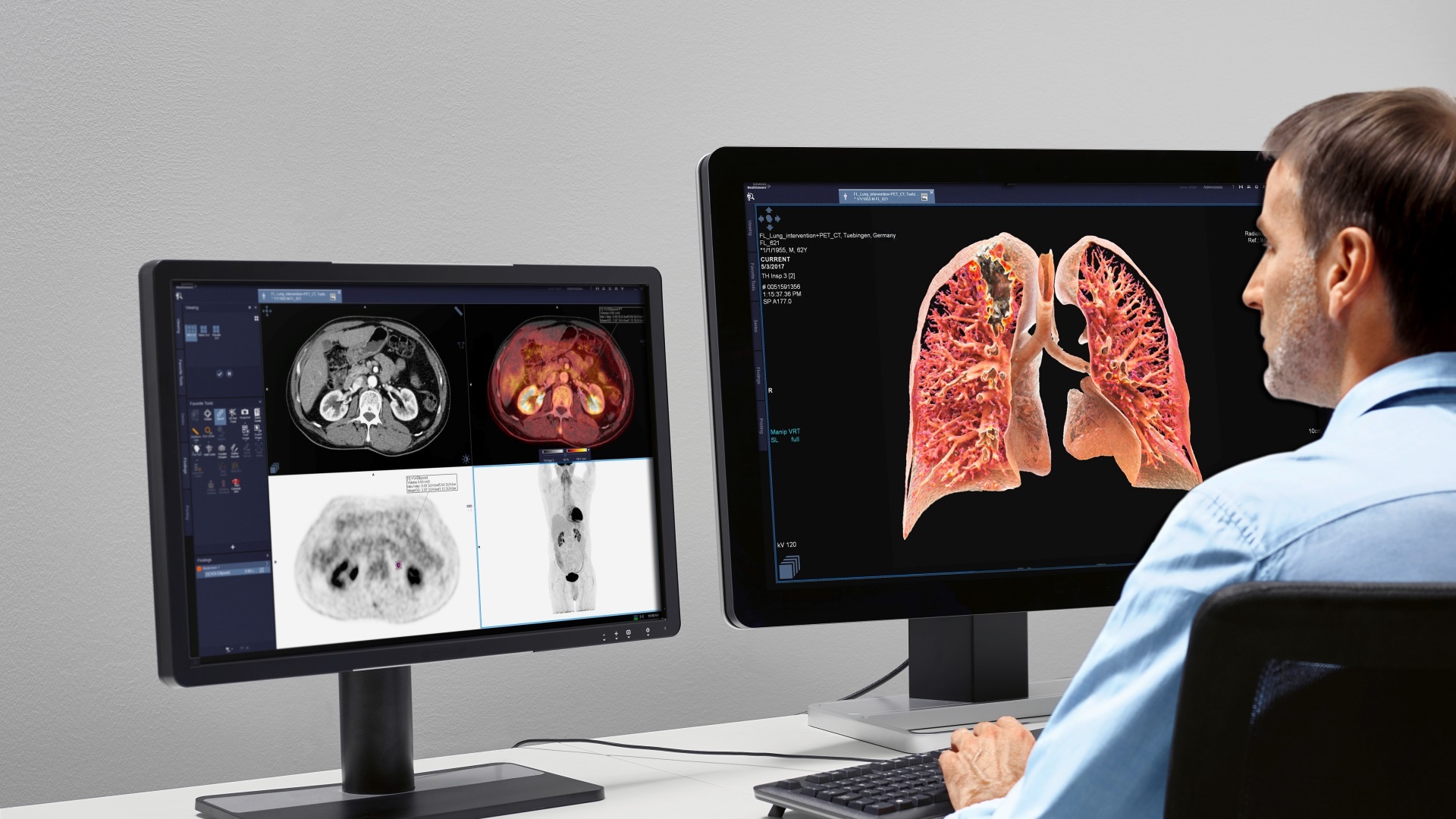
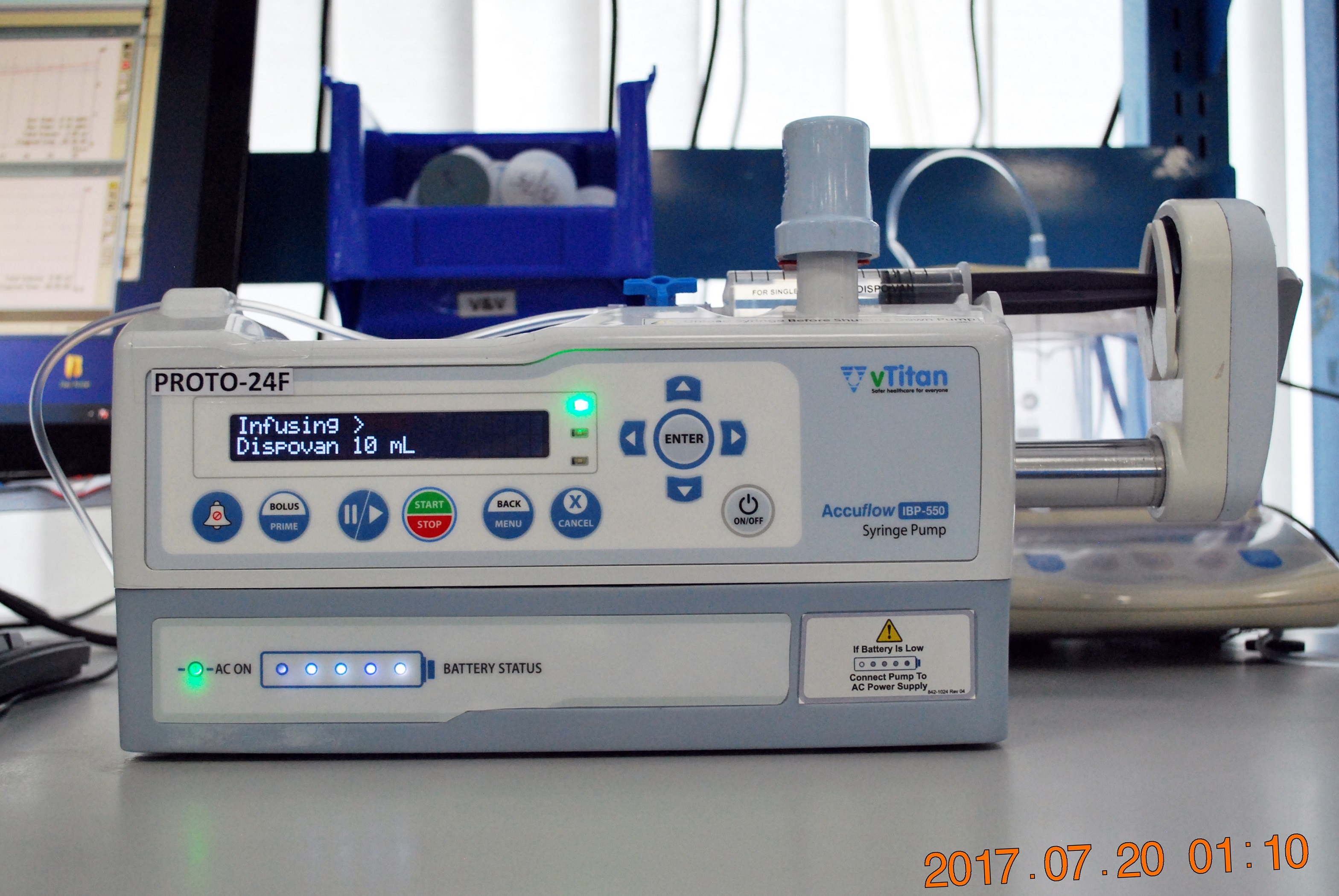
Medtronic has launched its PoleStar N30 Surgical MRI system in the USA as a device to help optimise tumour procedures during neurosurgery after getting US Food & Drug Administration (FDA) 510(K) clearance. The PoleStar Surgical MRI provides real-time imaging in the operating room, and targets and navigates accuracy despite any anatomy movement that may occur during a surgical procedure, while reducing the need for revision surgeries. According to the company, the PoleStar Surgical MRI system allows neurosurgeons to benefit from intra-operative MR imaging without the compromises inherent in other systems, such as extensive renovation of the operating room or restrictions on the surgeons’ choice of instruments. The PoleStar N30 system also received the Chicago Athenaeum Museum of Architecture and Design 2009 Good Design Award for global design excellence.

Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.
"Exciting news! Elets technomedia is now on WhatsApp Channels Subscribe today by clicking the link and stay updated with the latest insights!" Click here!