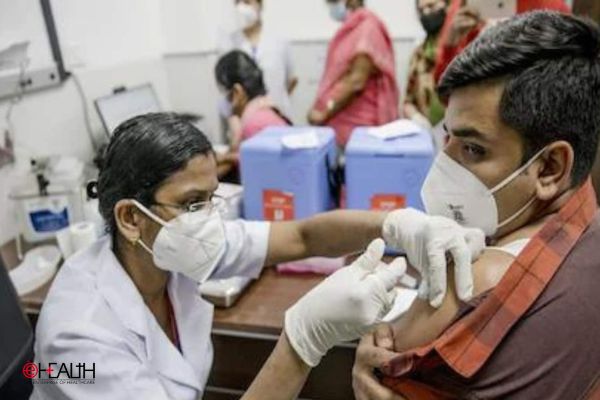
Post receiving conditional market approval from the Drug Controller General of India, Covaxin and Covishield will now be available in the regular market. People will be able to buy these vaccines from clinics and hospitals but not from regular medical shops. The approval has been granted only for the adult population.
The approval has been granted under the New Drugs and Clinical Trials Rules, 2019. Covaxin maker Bharat Biotech and Covishield maker Serum Institute of India shall submit data of ongoing clinical trials to DCGI every six months. Data will have to be updated on the CoWIN app as well.

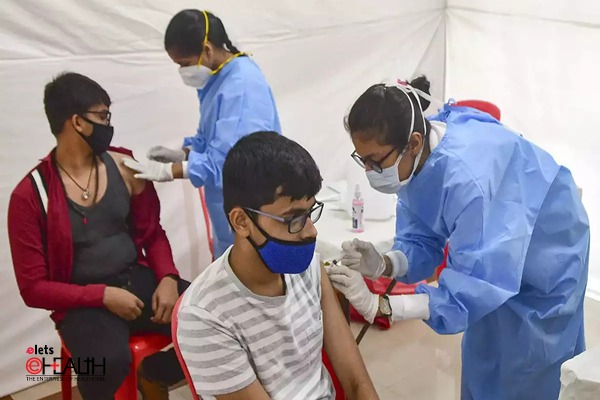
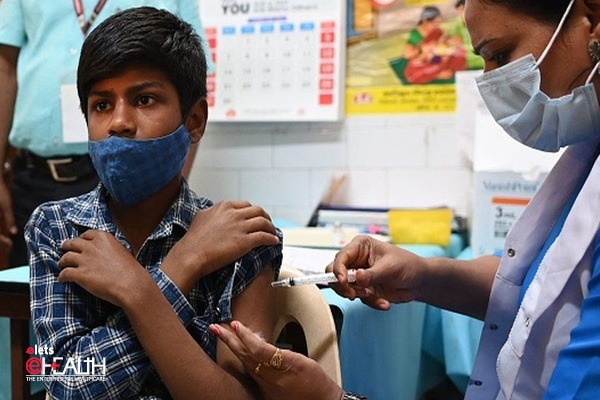
It is worth mentioning that the country’s COVID-19 vaccination coverage has crossed 163.84 crore dose with at least 72 per cent of India’s adult population fully vaccinated whereas around 52 per cent of children in the 15-18 years old age group have been administered the first dose.



Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.
"Exciting news! Elets technomedia is now on WhatsApp Channels Subscribe today by clicking the link and stay updated with the latest insights!" Click here!