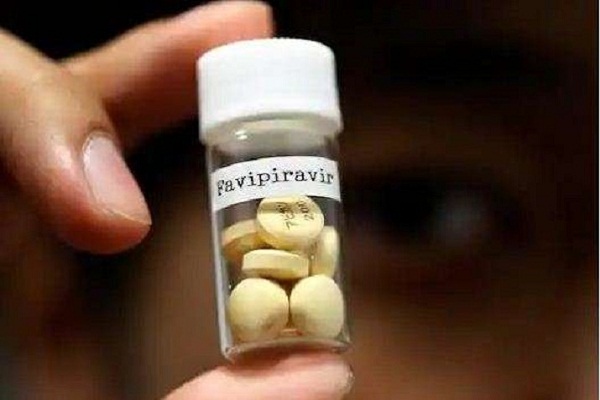
Drug firm FDC Ltd has launched two variants of the COVID-19 drug Favipiravir under the brand names PiFLU and Favenza. The Drug Controller General of India (DCGI) had earlier approved the use of Favipiravir, an off patent, oral antiviral drug that has been shown to quicken clinical recovery in COVID-19 patients with mild to moderate symptoms, FDC said in a statement.
“Early diagnosis and treatment will help in arresting the deteriorating condition of patients, and we will be working with the government and healthcare fraternity to make Favenza and PiFLU available across the country” FDC spokesperson Mayank Tikkha was quoted as saying by PTI report.

Also read: Lupin launches generic epilepsy tablets in US
Both the products are currently available across the country, the company said.

Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.












