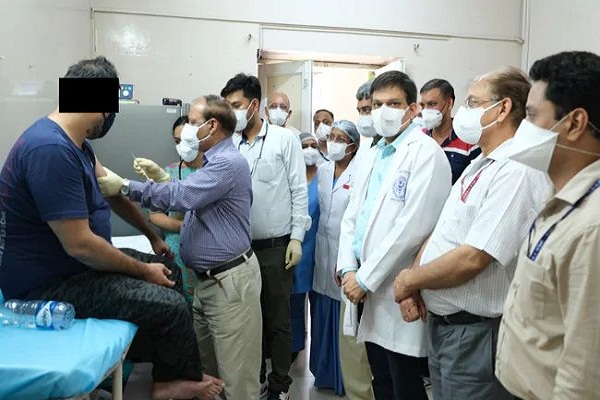
The phase-I human clinical trial of India’s first indigenously-developed vaccine against novel coronavirus, Covaxin, began at the AIIMS with the first dose of the injection given to a man, who is in his 30s.
Already, over 3,500 volunteers have registered themselves for the trial at AIIMS since last Saturday, of whom the screening of at least 22 people is underway, Dr. Sanjay Rai, Professor at the Centre for Community Medicine at AIIMS and the principal investigator of the study, told PTI.

“The first volunteer, a resident of Delhi, was screened two days ago and all his health parameters were found to be within the normal range. He also does not have any co-morbid conditions. “The first dose of 0.5 ml intramuscular injection was given to him around 1:30 p.m. No immediate side-effects have been observed so far. He was under observation for two hours and will be monitored for the next seven days,” Rai was quoted saying by the report.
Also read: India’s tryst with Covid-19 vaccine
Few more participants would be given the vaccine on Saturday after their screening reports come.

AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research for conducting phase I and II randomised, double-blind, placebo-controlled clinical trials of Covaxin.
In phase-I, the vaccine would be tested on 375 volunteers and the maximum of 100 of them would be from AIIMS. The second phase would include around 750 volunteers from all 12 sites together, Rai said
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.












