Announced by India’s Prime Minister, Shri Narendra Modi and UK’s Prime Minister, Sir Keir Starmer, India and the United Kingdom have successfully concluded negotiations for a landmark Free Trade Agreement (FTA), which according to the UK government is “the biggest and most economically significant bilateral trade deal the UK has done since leaving the EU”, while PM Modi has called the agreement “historic”.
The FTA aims to double bilateral trade in goods and services from $60 billion to $120 billion by 2030 and introduces several provisions impacting India’s healthcare industry, particularly in medical devices and pharmaceuticals. While the agreement offers opportunities for growth and collaboration, it also raises concerns among Indian industry leaders regarding regulatory standards and market dynamics.

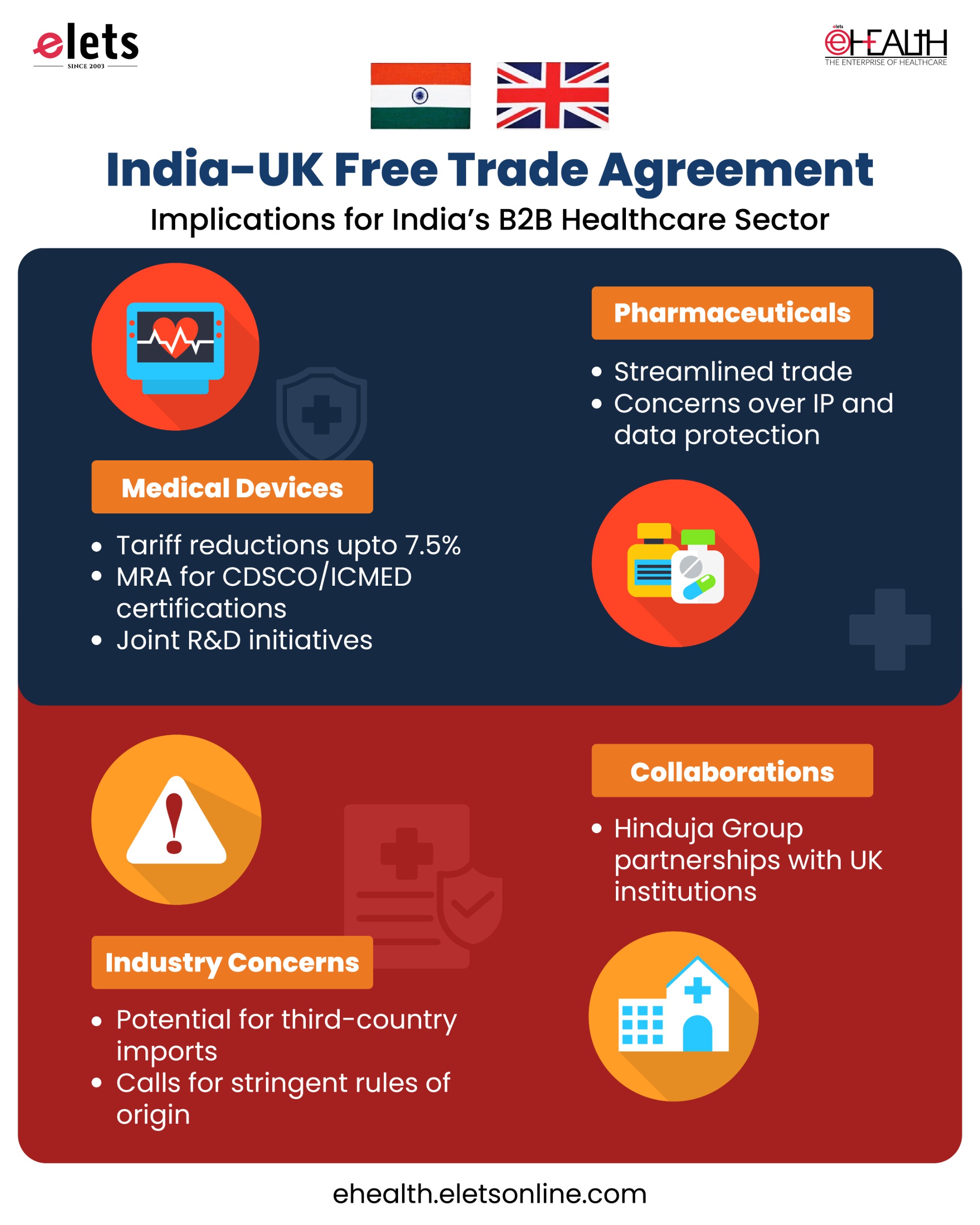


Key Provisions Affecting India’s Medical Devices Industry
- Tariff Reductions: The FTA reduces customs duties on critical medical devices, such as consumables, implants, and diagnostic equipment, which previously faced duties of up to 7.5% in India and 4.2% in the UK.
- Regulatory Harmonisation: A Mutual Recognition Agreement (MRA) framework is introduced to expedite market entry for Indian devices certified by the Central Drugs Standard Control Organisation (CDSCO) or the Indian Certification of Medical Devices (ICMED), reducing compliance costs and delays.
Key Provisions Affecting India’s Pharmaceutical Industry
- Trade Facilitation: The FTA’s provisions on pharmaceuticals are expected to streamline trade and reduce costs for healthcare providers in both countries, potentially leading to improved access to medical technologies and treatments.
- Tariff Reductions and Improved Regulatory Cooperation: The FTA includes tariff reductions and improved regulatory cooperation, which are expected to benefit UK exporters of medical devices and pharmaceutical products. India’s demand for such products is projected to rise, but no specific provisions regarding R&D, technology transfer, or Centres of Excellence are detailed in the agreement.
Industry Concerns and Safeguards on Free Trade Agreement
“Though we are still going through the fine print, strategically, the FTA reflects a geopolitical maturity: two democracies choosing to collaborate on healthcare innovation in an increasingly polarised global landscape. If followed through with rigour and vision, it could set a template for high-trust MedTech trade between developed and emerging economies.
One essential caveat: every FTA, including this one, must mandate clear disclosure of the actual site of manufacture for all imported products as mandated by India’s Central Drugs Standard Control Organisation (CDSCO), which requires separate registration of both the legal and actual manufacturers. The CDSCO is duty-bound to implement this provision rigorously, honestly and consistently. This is critical to prevent trans-shipment from third countries or undisclosed locations. Some geographies lack such regulatory rigour, and even within a country, manufacturing plants can vary greatly in standards. Transparent, unambiguous disclosure ensures legal compliance, protects patient safety through true traceability, and upholds a level playing field for all companies and especially those which adhere to a high level of regulatory compliance,” said Pavan Choudary, Chairman of the Medical Technology Association of India (MTaI).

“While this a path breaking initiative, FTAs in past have been detrimental to the medical devices sector and only made us more important dependent and less Atmaribhar – we had explained our concerns to Department of Pharma and Dept Of Commerce.
Our concerns had been that for this to be a mutually beneficial deal we needed fast track regulatory approval based on ICMeD – ISO certification to over come non tariff measures put in by UK and to safeguard Indian manufacturers from rerouted products from China etc via UK as purportedly made in UK or UK country of Origin products even if wholly made by a “UK based legal “ (and not actual manufacturer ) manufacturer in a 3 rd country. We are not afraid of competing with UK-made products. We had sought Dept Of Commerce to ensure that goods coming under UK FTA benefits to India needed to have over 35% value added in UK with change of tariff heading of inputs to qualify as manufactured in UK- we welcome UK medical devices into India as long as these do not undermine make in India of medical devices in India. We are seeking finer details to enable us to make an informed comment, “ stated Rajiv Nath, Forum Coordinator, AiMeD.
“With the recent UK-India FTA reducing import tariffs on advanced medical equipment, we indeed foresee significant positive implications for healthcare providers. The lowered import costs on high-end medical technologies — including robotic surgical systems and precision oncology equipment — have the potential to directly benefit both providers and patients. At HCG Manavata, we are committed to delivering cutting-edge cancer care, and reduced capital costs for advanced surgical technologies will allow us to enhance access to sophisticated procedures such as robotic-assisted surgeries. Over time, as these technologies become more accessible and costs decline, we anticipate a narrowing gap between the pricing of robotic-assisted surgeries and conventional cancer surgeries. This will make advanced cancer treatments more affordable and accessible to a broader segment of patients, particularly in tier-II and tier-III cities, where affordability has historically been a barrier. Moreover, this development is poised to elevate the standard of cancer care in India. Greater availability of advanced equipment will enable more precise, minimally invasive surgeries with shorter recovery times and better outcomes, aligning Indian cancer care more closely with global best practices. We view this as an opportunity not just to upgrade technology but also to expand equitable access to high-quality cancer care, furthering our mission of bringing comprehensive oncology services to every patient in need,” says Prof. Dr. Raj Nagarkar, Chief of Surgical Oncology & Robotic Services & Managing Director, HCG Manavata Cancer Centre (HCGMCC) & Hospitals.
Also Read :- Strengthening Gen AI’s role in Healthcare
Implementation Timeline
- Legal scrubbing to take ~3 months.
- Followed by signing, approval from the Union Cabinet (India) and the UK Parliament.
- Implementation is expected within a year on a mutually agreed date.
Economic Impact
- Boost to Bilateral Trade: Expected to add $34 billion/year to trade volume by 2040.
- Job Creation: Especially in labour-intensive sectors in India like textiles, leather, and services.
- Innovation & Investment: Promotes technology transfer and cooperation in clean energy, life sciences, AI, and manufacturing.
The India–UK Free Trade Agreement represents a significant step forward in bilateral trade relations, offering notable opportunities for India’s healthcare sector, including reduced tariffs on medical devices, potential cost savings, and improved access to advanced technologies. However, concerns remain around regulatory harmonisation, the risk of trans-shipment from third countries, and the potential impact on domestic manufacturers if adequate safeguards are not enforced. While the agreement lays the groundwork for deeper collaboration, its success will depend on robust implementation, transparent compliance mechanisms, and continued engagement with industry stakeholders to ensure that India’s long-term healthcare and manufacturing interests are protected.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.
"Exciting news! Elets technomedia is now on WhatsApp Channels Subscribe today by clicking the link and stay updated with the latest insights!" Click here!