
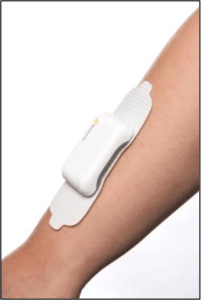
 Bioness, Inc., a leading provider of innovative technologies helping people regain mobility and independence, has announced the commercial availability of StimRouter. StimRouter is cleared by the FDA to treat chronic pain of peripheral nerve origin. It is a minimally invasive neuromodulation medical device consisting of an implanted lead, external pulse transmitter (EPT) and conductive electrode, controlled by a small, hand-held wireless control unit.
Bioness, Inc., a leading provider of innovative technologies helping people regain mobility and independence, has announced the commercial availability of StimRouter. StimRouter is cleared by the FDA to treat chronic pain of peripheral nerve origin. It is a minimally invasive neuromodulation medical device consisting of an implanted lead, external pulse transmitter (EPT) and conductive electrode, controlled by a small, hand-held wireless control unit.
The commercial launch of StimRouter for peripheral pain positions Bioness to strategically build on the success of our neuromodulation portfolio. In addition to pain applications, we started a pilot study for overactive bladder in February and we anticipate expanding to other commercial markets in the future, said Todd Cushman, President and CEO, Bioness.

The company makes external and implantable functional electrical stimulation systems, robotic systems and software-based therapy programs providing functional and therapeutic benefits for individuals affected by pain, central nervous system disorders and orthopaedic injuries.
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.












