Sysmex, Transasia introduce products for Clinical Research sector
 Sysmex, the world leader in Haematology, in partnership with Transasia Bio-Medicals introduced recently the XT-1800i V & XT-2000i V (Veterinary mode), a fully automated Haematology analyser for High Quality Animal Blood testing. This instrument, it is said will be highly beneficial to India’s growth in the Contract Clinical Research sector and Pharmaceutical R&D.
Sysmex, the world leader in Haematology, in partnership with Transasia Bio-Medicals introduced recently the XT-1800i V & XT-2000i V (Veterinary mode), a fully automated Haematology analyser for High Quality Animal Blood testing. This instrument, it is said will be highly beneficial to India’s growth in the Contract Clinical Research sector and Pharmaceutical R&D.

A lot of CROs and Pharmaceutical companies in India are working on development of new Drugs both independently and in collaboration with certain MNCs. There are very strict regulatory norms both in India and the US, which need to be followed. Animal testing is also mandatory to check the effectiveness of different compounds and their side effects. XT-1800i V and XT-2000i V Vet Analyser offers superior diagnostics for animal blood analysis thereby ensuring high quality standards to support clinical trials and drug discovery.
The unique feature of XT-1800i V and XT-2000i V Vet Analyser is its ability to analyse as many species as can be defined by the users themselves. The use of principle of Advanced Fluorescence flowcytometry ensures Efficient Quality Control. It also ensures the ability to trace and record all data without any possibility of overwriting the original data, which is in compliance with U.S. FDA.
XT-1800i V and XT-2000i V Vet Analyser is a must for all veterinary universities, research labs, Veterinary Hospitals, Pharmaceutical Companies and Toxicological Labs involved in Clinical trials as it is Low maintenance and Robust analyser. Also most of the Companies involved in Clinical trials work in partnership with companies operational at the global level and have work going on simultaneously at different centers. They need to compile data in real time, which only Sysmex can offer by its online Quality Control of Sysmex Network Communication System (SNCS) – an Internet based online customer service, which allows maintainence of quality on Sysmex hematology analysers. Above all the direct benefit to all will be a better health and access to a subsidised Healthcare.

Escorts introduces 3D Trans Esophageal Echo in India
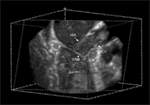 Escorts Heart Institute and Research Centre Ltd. (EHIRCL), amongst the world’s largest standalone cardiac institutions, recently organised a symposium on ‘Emerging Techniques in Echocardiography’ at India Habitat Centre, New Delhi. Doctors from across Delhi and NCR came together for the session on the revolutionary 3D Trans Esophageal Echocardiography (3D TEE). Eminent cardiac specialist from EHIRCL like Dr. Ashok Seth, Dr. Savitri Shrivastava, Dr. Ashok, K Omar and Dr. Sameer Srivastava addressed the sessions sharing various case studies.
Escorts Heart Institute and Research Centre Ltd. (EHIRCL), amongst the world’s largest standalone cardiac institutions, recently organised a symposium on ‘Emerging Techniques in Echocardiography’ at India Habitat Centre, New Delhi. Doctors from across Delhi and NCR came together for the session on the revolutionary 3D Trans Esophageal Echocardiography (3D TEE). Eminent cardiac specialist from EHIRCL like Dr. Ashok Seth, Dr. Savitri Shrivastava, Dr. Ashok, K Omar and Dr. Sameer Srivastava addressed the sessions sharing various case studies.
Dr. Navin C. Nanda, Director of Echocardiography and Heart Stations, University of Alabama, Birmingham (USA), spearheaded the session on clinical usefulness of the new 3D Trans Esophageal Echocardiography in the field of Heart treatment. Dr. Nanda was the first recipient of the Tufts University Award in Echocardiography and in 2006 received the Ellis Island Medal of Honour. 3 D TEE is the latest series of echocardiography imaging; this is a technique wherein ultrasound of the heart is done using the esophagus (food pipe) and stomach as a window vis-�-vis conventional trans thoracic echo, which is obtained by putting the probe across the surface of the chest. 3D TEE offers benefits like superior quality heart images, faster image acquisition for the heart treatment. Escorts Heart Institute and Research Centre Ltd. (EHIRCL), is one of the first to get this technology in India.
Centre plans drug stores in all districts in India
The government has decided to set up a retail network of drug stores across the country in public-private partnership that would sell 350 essential medicines at half the rate of its branded substitute. The project, being launched in 15 states in the first phase, would ultimately ensure at least one such store in every district of the country. The move aims at ending company-chemist nexus, which tries to push costlier branded medicines to customers in the name of substitutes.
These stores would sell only good-quality generic medicines. Generic formulations are identical to their branded counterpart in terms of dose, strength, efficacy and use.
It is learnt from official sources that the government has asked industry associations like Indian Drug Manufacturers Association, Organisation of Pharmaceutical Producers Of India, Indian Pharmaceutical Association, Confederation of Indian Pharmaceutical Industry and SPIC to join hands with it in its endeavour. Apart from private players, the government has also approached NGOs, chemist associations and state governments to participate in the project. While state governments are being sought to provide space for opening shops, chemist associations and NGOs are likely to play a major role in managing and promoting these shops in future.
There is a global shift towards use of generics as governments world-wide are making efforts to bring down escalating��healthcare budgets. India has achieved a strong foothold in the global generics market. While in 2002 Indian companies accounted for less than 7% of all generic drugs approved for marketing by the US Food and Drug Administration, they accounted for over 20% in 2006.
CSIR launches collaborative research for anti-TB drugs
 India recently launched a unique collaborative programme to discover drugs for infectious diseases common to the developing countries.
India recently launched a unique collaborative programme to discover drugs for infectious diseases common to the developing countries.
The ‘Open Source Drug Discovery’ (OSDD) programme, launched by the Council of Scientific and Industrial Research (CSIR), aims to build a consortium of global researchers and bypass the patent regime, which makes drugs expensive.
To begin with OSDD, a brainchild of CSIR Director General Samir K. Brahmachari, has taken up research on discovering new drugs for treatment of tuberculosis, a field in which no major advancement in treatment has emerged since 1960.
“The normal process of drug discovery, through the patent regime, has not worked very well for diseases in our part of the world,” Science and Technology Minister Kapil Sibal told reporters here.
Inspired by open source movements like Linux and the human genome sequencing project, OSDD seeks to expand resources for research manifold by allowing collaboration among voluntary researchers.
CSIR has set up a website – www.osdd.net – as a platform for collaborative research, data on pathogens, tools for data analysis, and discussion forum for members to share ideas and projects for students to participate in drug discovery.
Laboratory experiments during this process will be carried out at CSIR-sponsored centres. CSIR has earmarked INR 150 crore for the OSDD project under the 11th Plan and an equivalent amount of funding is expected to be raised from international agencies and philanthropists.
Balco to build INR 300 cr cancer hospital in Chhattisgarh
The Bharat Aluminium Company Ltd (Balco), which is a part of the Vedanta Group, will spend INR 300 crore in building a cancer hospital and research centre here, officials said.
“The state government has given a 41 acre plot of land free of cost to Balco at village Saddu in capital Raipur to build the cancer hospital,” an official said. The land has been handed given to the company on lease for a 30-year period.
Balco will develop the hospital, the first of its kind in the state, within 18 months of the beginning of construction in November this year.
India’s ninth largest state in terms of area, Chhattisgarh has an estimated population of 20.08 million but lacks medical and health care facilities. Balco has assured the government that it will treat poor people for free at its hospital.
Govt puts clinical research approvals on fast lane
 The government has begun speeding up approvals in the area of clinical research, which is set to boom in India, say top industry sources. The clinical research industry in India is currently USD 200 million, but is expected to reach USD 1.5 billion in just two years.
The government has begun speeding up approvals in the area of clinical research, which is set to boom in India, say top industry sources. The clinical research industry in India is currently USD 200 million, but is expected to reach USD 1.5 billion in just two years.
The government is also planning on introducing fingerprinting of clinical trial volunteers to ensure better data from the trials and also regularise insurance claims by these volunteers.
Currently, only Indian pharma companies are allowed to conduct clinical trials from phase zero. Foreign firms are only allowed to conduct phase 2 and 3 trials in the country. But the DCGI plans to allow foreign firms to conduct phase zero and 1 trials also. This could definitely provide a boost to the industry and help it reach the projected number of USD 1.5 billion soon. The removal of service tax for CROs and customs duty on all imported drugs used in clinical trials has helped give this industry a boost. It has been noted that the clinical research industry is growing faster than the pharmaceutical industry today and research firms are getting into long-term partnering deals.
A Merill Lynch report has stated that India is a more economically viable destination to conduct clinical trials. The overall cost of conducting trials in the US is 2.5 times the cost in India.
The increase in health and allied infrastructure and the implementation of good clinical practices (GCP) guidelines, is another reason why companies across the globe are looking at India as an attractive destination for clinical research. KPMG’s latest pharma report states that according to the US National Institutes of Health, about 272 trials are actively recruiting patients in India. Of this, 60% are phase 3 trails. In March 2008, about 80 hospitals (both government and private) were estimated to be involved in clinical trails apart from the 290 clinical research organisation in the country.
Indian companies have shifted from paper trials to electronic data capture (EDC) trials. But even this, industry sources say, needs to be improved to compete with countries like China, which is also an aggressive player in the clinical research space. 
Be a part of Elets Collaborative Initiatives. Join Us for Upcoming Events and explore business opportunities. Like us on Facebook , connect with us on LinkedIn and follow us on Twitter , Instagram.


